 |
The Food and Drug Administration assumed something of a defensive crouch in granting on Oct. 12 its first-ever marketing authorization for an e-cigarette, R.J. Reynolds Vapor Company’s Vuse Solo tobacco-flavored product.
The federal agency said its action does not mean that the product is safe or “FDA approved” because “all tobacco products are harmful and addictive and those who do not use tobacco products should not start.”
In the FDA’s view, Vuse Solo is less harmful than smoking cigarettes. That’s walking a very fine line. Anti-tobacco activists blasted the FDA authorization.
The FDA credited RJR’s data for demonstrating that “its tobacco-flavored products could benefit addicted adult smokers who switch to those products—either completely or with a significant reduction in cigarette consumption—by reducing their exposure to harmful chemicals,” Mitch Zeller, director of FDA’s Center for Tobacco Products, said in a statement.
In granting marketing authorization, the FDA said it considered the risks and benefits to the general population, including young people.
It determined that the potential benefit to smokers who switch completely or cut their cigarette use outweighed the risk of hooking kids on e-cigarettes. Talk about throwing young people under the bus.
The FDA said young people use fruit, candy and mint-flavored products, rather than tobacco-flavored, and it did issue “marketing denial orders” for five RJR flavors that it no longer markets.
The FDA claims it has imposed marketing restrictions on RJR to reduce the potential for youth exposure to tobacco e-cigarettes.
It threatens to yank Vapor Solo’s authorization if it finds that its marketing results in “a significant increase in youth initiation.”
The FDA is still reviewing three RJR e-cigarettes that the company says share the “foundational science” of Vuse Solo and is confident in the quality of their applications.
RJR may have more PR victories ahead at the expense of the FDA.
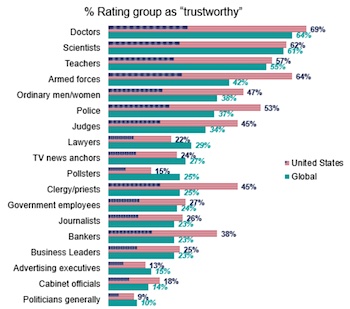 |
The advertising business is desperate for a PR makeover, according to a Ipsos poll on trustworthiness released Oct. 12.
Of the 18 groups measured by Ipsos, Americans said only politicians (nine percent) are less trustworthy than ad people (13 percent).
Though battered during the Trump regime as being “enemies of the people,” journalists, at 26 percent, managed to top business leaders (25 percent), cabinet officers (18 percent), and pollsters (15 percent).
Doctors (69 percent), armed forces (64 percent) and scientists (62 percent) are the three most trusted groups.
The poll found some big trustworthy gaps between Americans and the rest of the world.
Though America loves its soldiers, the military is trusted by only 42 percent by the rest of the world.
Priests/clergy get a 45 percent trustworthy score here and only 25 percent elsewhere.
Americans give bankers a 38 percent trust score, while they are trusted by only 23 percent of people outside the US.
Ipsos did not rank PR people, who undoubtedly would have rivaled doctors in the trust category.


 Southern governors claim they know what's best for their working class, and it's not pay raises... A Ukrainian human rights group played a key role in convincing House Speaker Mike Johnson to hold a vote to send arms to Ukraine, Israel and Taiwan... Trump Media & Technology Group blames short-selling and not lousy outlook for its stock slump.
Southern governors claim they know what's best for their working class, and it's not pay raises... A Ukrainian human rights group played a key role in convincing House Speaker Mike Johnson to hold a vote to send arms to Ukraine, Israel and Taiwan... Trump Media & Technology Group blames short-selling and not lousy outlook for its stock slump. The techniques deployed by OJ Simpson's defense team in the 'trial of the century' served as a harbinger for those used by Donald Trump... People worry about the politicization of medical science just as much as they fret about another pandemic, according to Edelman Trust Barometer... Book bans aren't restricted to red states as deep blue Illinois, Connecticut and Maryland challenged at least 100 titles in 2023.
The techniques deployed by OJ Simpson's defense team in the 'trial of the century' served as a harbinger for those used by Donald Trump... People worry about the politicization of medical science just as much as they fret about another pandemic, according to Edelman Trust Barometer... Book bans aren't restricted to red states as deep blue Illinois, Connecticut and Maryland challenged at least 100 titles in 2023. The NBA, which promotes legalized gambling 24/7, seems more than hypocritical for banning player for placing bets... Diocese of Brooklyn promises to issue press release the next time one of its priests is charged with sexual abuse... Truth Social aspires to be one of Donald Trump's iconic American brands, just like Trump University or Trump Steaks or Trump Ice Cubes.
The NBA, which promotes legalized gambling 24/7, seems more than hypocritical for banning player for placing bets... Diocese of Brooklyn promises to issue press release the next time one of its priests is charged with sexual abuse... Truth Social aspires to be one of Donald Trump's iconic American brands, just like Trump University or Trump Steaks or Trump Ice Cubes. Publicis Groupe CEO Arthur Sadoun puts competition on notice... Macy's throws in the towel as it appoints two directors nominated by its unwanted suitor... The Profile in Wimpery Award goes to the Ford Presidential Foundation for stiffing American hero and former Wyoming Congresswoman Liz Cheney.
Publicis Groupe CEO Arthur Sadoun puts competition on notice... Macy's throws in the towel as it appoints two directors nominated by its unwanted suitor... The Profile in Wimpery Award goes to the Ford Presidential Foundation for stiffing American hero and former Wyoming Congresswoman Liz Cheney. JPMorgan Chase chief Jamie Dimon's "letter to shareholders" is a must-read for PR people and others interested in fixing America and living up to its potential... Get ready for the PPE shortage when the next pandemic hits... Nixing Netanyahu. Gaza carnage turns US opinion against Israel's prime minister.
JPMorgan Chase chief Jamie Dimon's "letter to shareholders" is a must-read for PR people and others interested in fixing America and living up to its potential... Get ready for the PPE shortage when the next pandemic hits... Nixing Netanyahu. Gaza carnage turns US opinion against Israel's prime minister.


 Have a comment? Send it to
Have a comment? Send it to 
No comments have been submitted for this story yet.